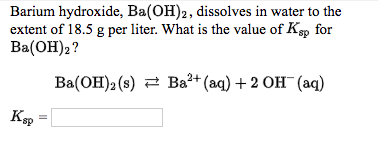40. 50ml H2O is added to a 50ml solution of Ba(OH)2 of strenth 0.01M. The pH value of the resuultingsolution will be

OneClass: 3. Consider the reaction: 2HCl(aq) + Ba(OH)2(a) â†' BaCl2(aq) + 2 H2O(l) Δ --118 kl A) Cal...

Ba + H2O =Ba(OH)2 +H2 Balanced Equation||Barium and Water=Barium hydroxide plus H2 Balanced Equation - YouTube

рассчитайте по термохимическому уравнению BaO+H2O=Ba(OH)2+73 кДж сколько выделяется энергии - Школьные Знания.com
BALANCE THIS GIVEN EQUATION BY THE HELP OF THE ALTERNATE WAY OF BALANCING NOT THE TRADITIONAL WAY BY SHOWING THE APPROPRIATE STEPS ELABORATELY. EQN : Ba(OH)2 + NH4Cl —> BaCl2 + NH3 + H2O