Thermal characteristics enhancement of Na2HPO4⋅12H2O/expanded graphite form-stable composite phase change material by the cationic surfactant modification - ScienceDirect

Preparation and characterization of Na2HPO4·12H2O@polymethyl methacrylate nanocapsule for efficient thermal energy storage - ScienceDirect

Enhancing the Heat Storage Performance of a Na2HPO4·12H2O System via Introducing Multiwalled Carbon Nanotubes | ACS Omega

Super absorbent polymer as support for shape-stabilized composite phase change material containing Na2HPO4·12H2O–K2HPO4·3H2O eutectic hydrated salt - ScienceDirect

10101-89-0, 380.12, Sodium Phosphate Tribasic, Dodecahydrate, Crystal, Reagent, ACS - 6NNZ0|S1410-12KG - Grainger

For the equilibrium SrCl2· 6H2O(s) SrCl2· 2H2O(s) + 4H2O(g) the equilibrium constant Kp = 16 × 10^-12 atm^4 at 1^0 C. If one litre of air saturated with water vapour at 1^0

10101-89-0, 380.12, Sodium Phosphate Tribasic, Dodecahydrate, Crystal, Reagent, ACS - 6NNZ1|S1410-2.5KG - Grainger
![SOLVED: 1.) calculate how many grams of disodium hydrogen phosphate dodecahydrate [Na2HPO4 * 12H2O] ans water should be used to prepare 27 grams of 4.5 % solution of this salt. 2.) How SOLVED: 1.) calculate how many grams of disodium hydrogen phosphate dodecahydrate [Na2HPO4 * 12H2O] ans water should be used to prepare 27 grams of 4.5 % solution of this salt. 2.) How](https://cdn.numerade.com/ask_previews/d3e9cad5-07e7-4af0-b983-9e3d75ec767e_large.jpg)
SOLVED: 1.) calculate how many grams of disodium hydrogen phosphate dodecahydrate [Na2HPO4 * 12H2O] ans water should be used to prepare 27 grams of 4.5 % solution of this salt. 2.) How
![10039-32-4・Disodium Hydrogenphosphate 12-Water・190-02833・198-02834・192-02837・192-02832・194-02831・196-02835[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 10039-32-4・Disodium Hydrogenphosphate 12-Water・190-02833・198-02834・192-02837・192-02832・194-02831・196-02835[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/05/192-02837.png)
10039-32-4・Disodium Hydrogenphosphate 12-Water・190-02833・198-02834・192-02837・192-02832・194-02831・196-02835[Detail Information] | [Common Chemicals & Lab Tools]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation
![10039-32-4・Dibasic Sodium Phosphate Hydrate・043-34387・047-34385[Detail Information] | [Pharma Manufacturing & QC]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 10039-32-4・Dibasic Sodium Phosphate Hydrate・043-34387・047-34385[Detail Information] | [Pharma Manufacturing & QC]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/05/047-34385.png)
10039-32-4・Dibasic Sodium Phosphate Hydrate・043-34387・047-34385[Detail Information] | [Pharma Manufacturing & QC]|Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation

Supercooling Suppression and Thermal Conductivity Enhancement of Na2HPO4· 12H2O/Expanded Vermiculite Form-Stable Composite Phase Change Materials with Alumina for Heat Storage,ACS Sustainable Chemistry & Engineering - X -MOL

Synchrotron XRD patterns of frozen sodium phosphate buffer solution... | Download Scientific Diagram
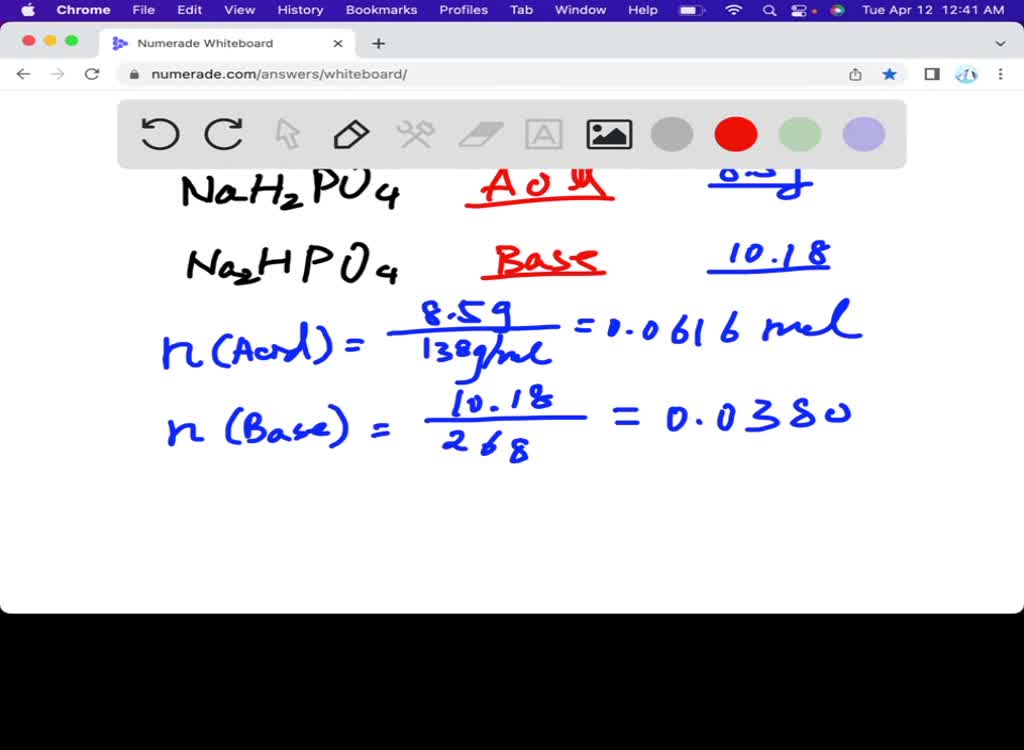
SOLVED: Calculate the pH of a buffer solution prepared by dissolving 8.5g of NaH2PO4 and 10.18g NA2HPO4 in 1 L water. NaH2PO4 * H2O MW= 138; Na2HPO4 * 7 H2O MW= 268 pka= 7.21.