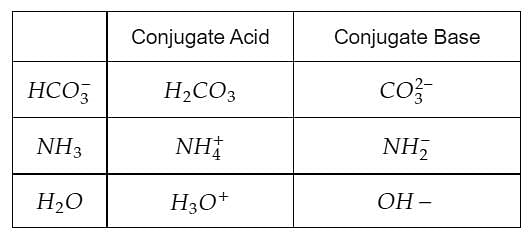
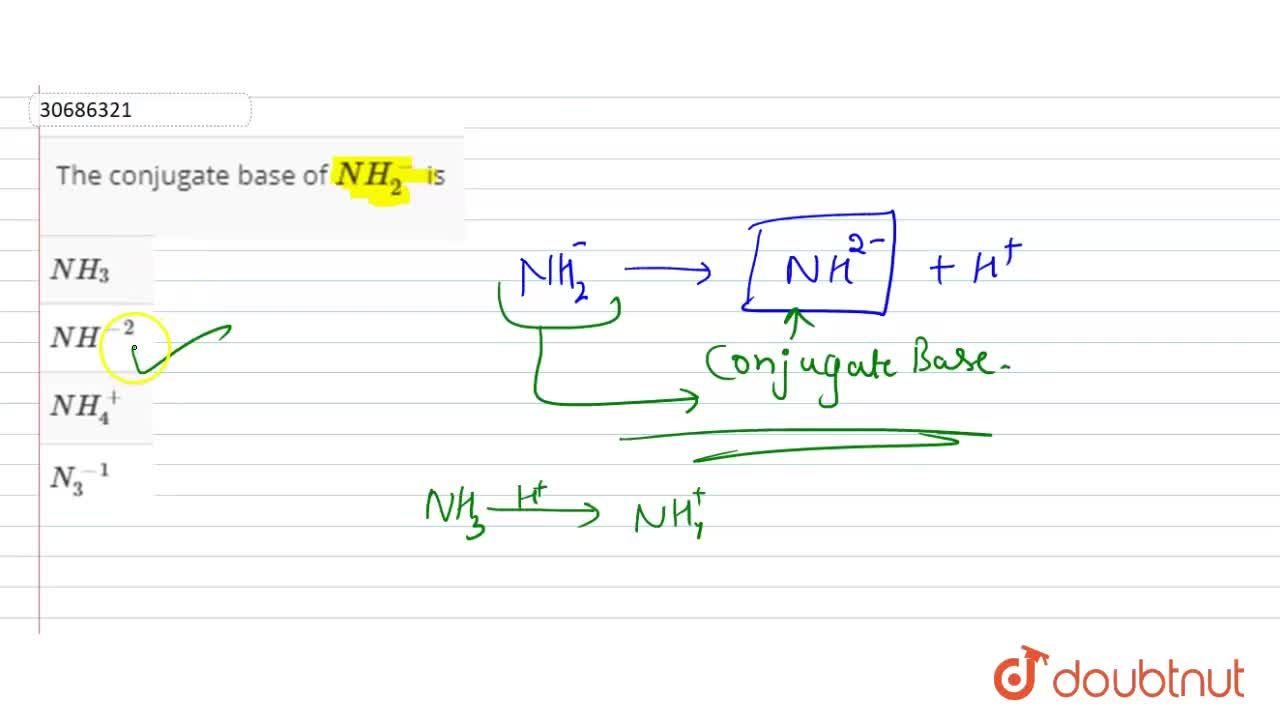
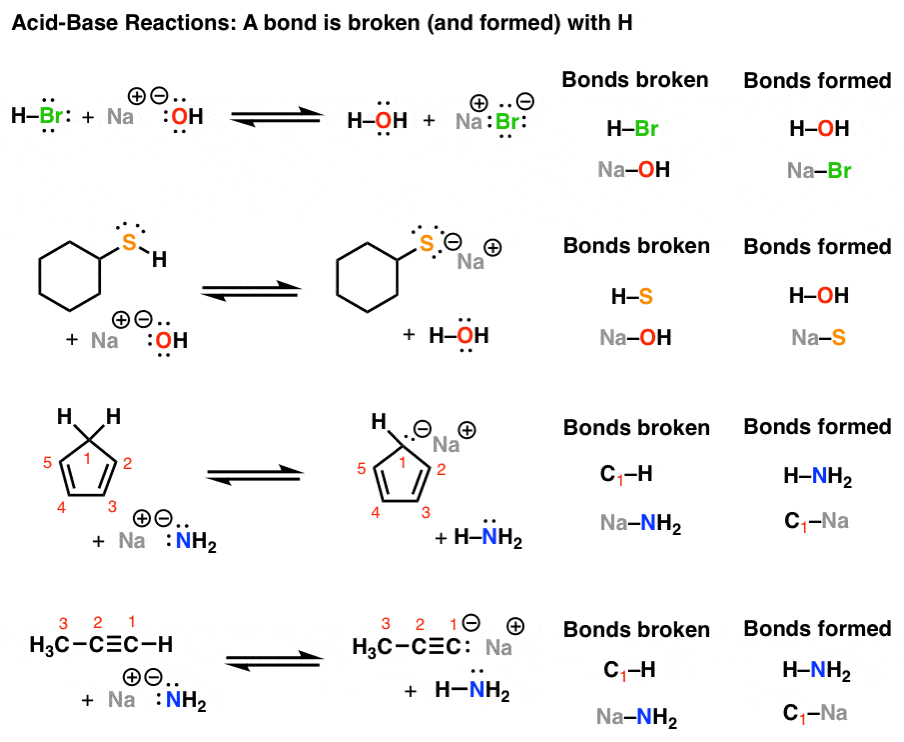
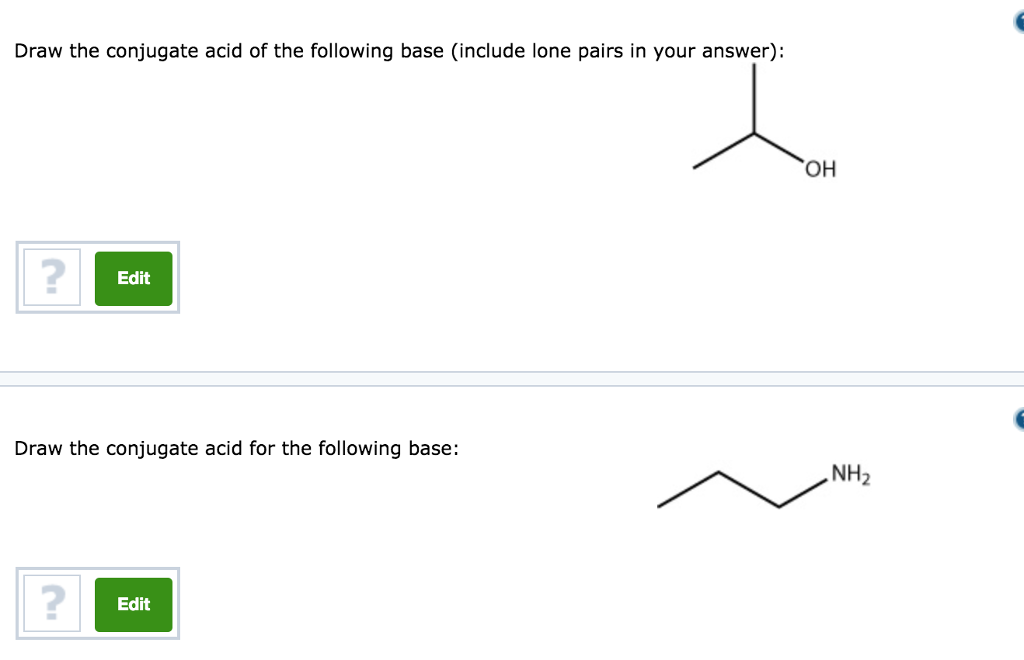
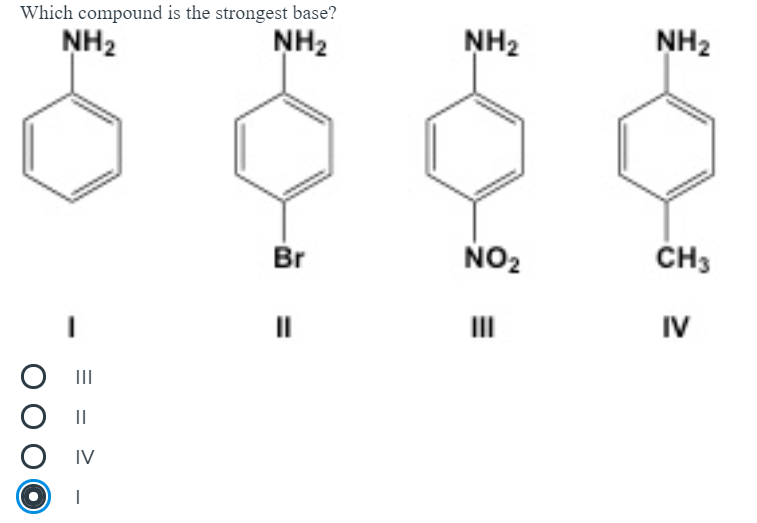
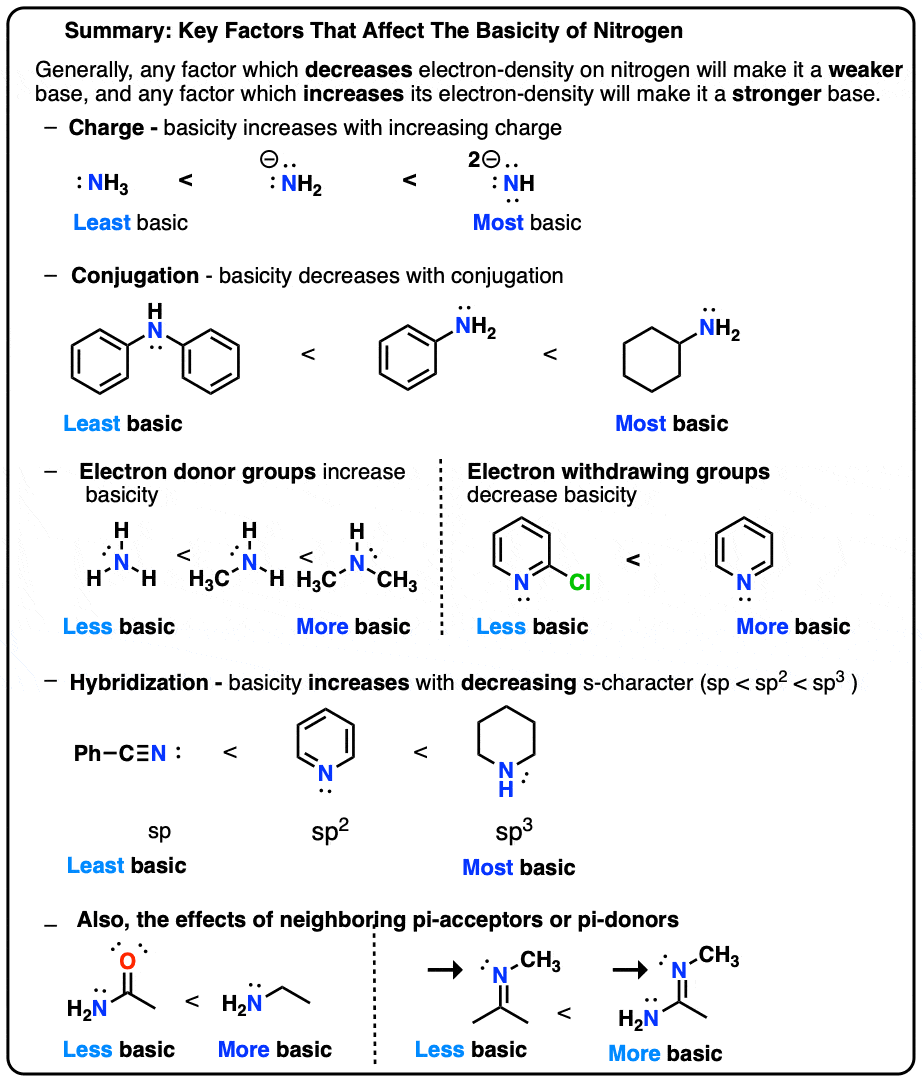
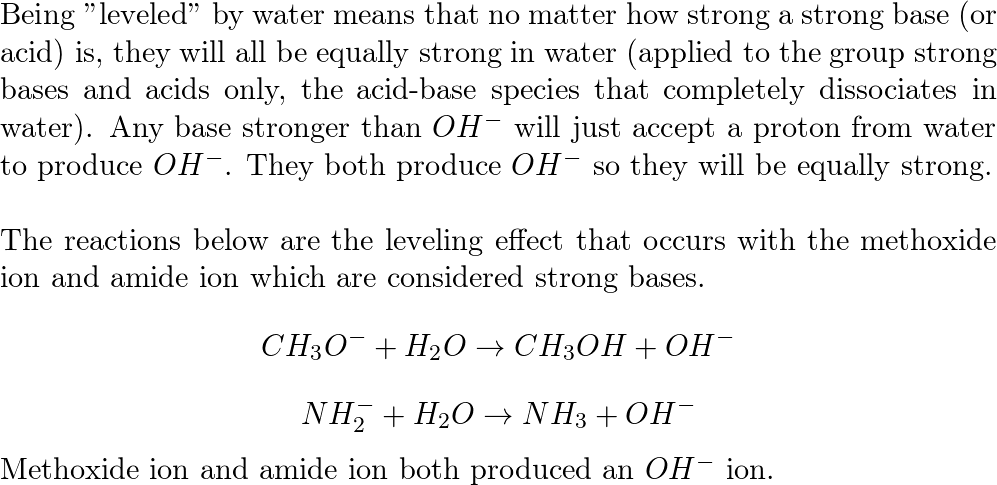The correct decreasing order of basic strength of the following species is . H2O, NH3,OH^(-), NH2^(-)

Tröger's Base Polyimide Hybrid Membranes by Incorporating UiO-66-NH2 Nanoparticles for Gas Separation | Industrial & Engineering Chemistry Research

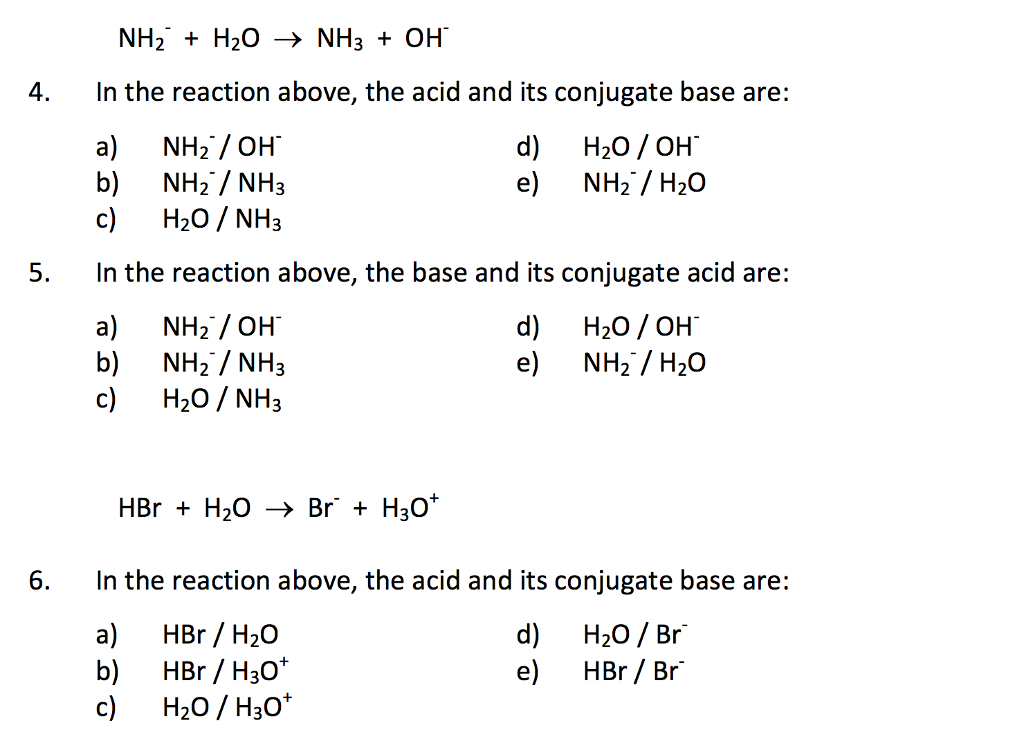
OneClass: Questions 3 and 4. Consider the reaction shown below: NH2-(ag) + H2O(l) ê·¼ NH3(gg) + OH-(a...
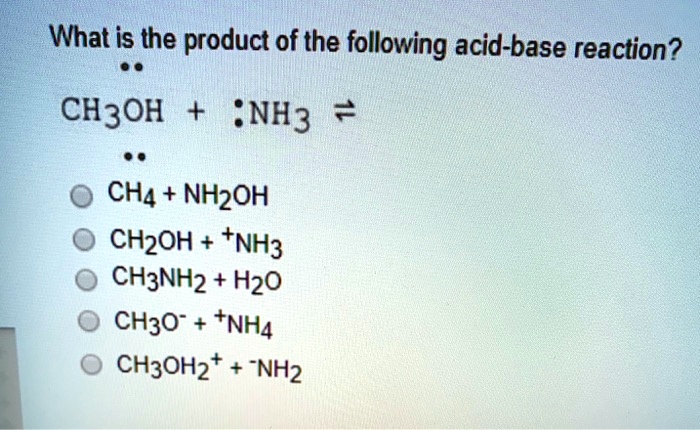
SOLVED: What is the product of the following acid-base reaction? CH3OH :NH3 2 CHA - NHZOH CH2OH +NH3 CH3NH2 Hzo CH3O" +NHA CH3OH2" NH2