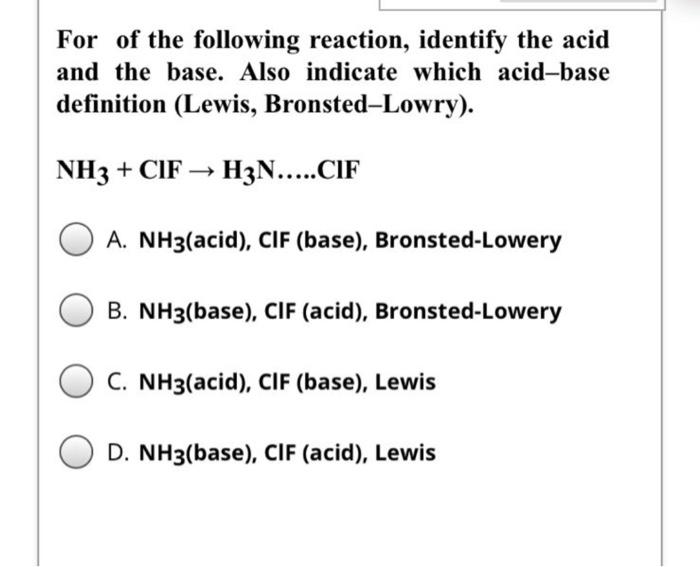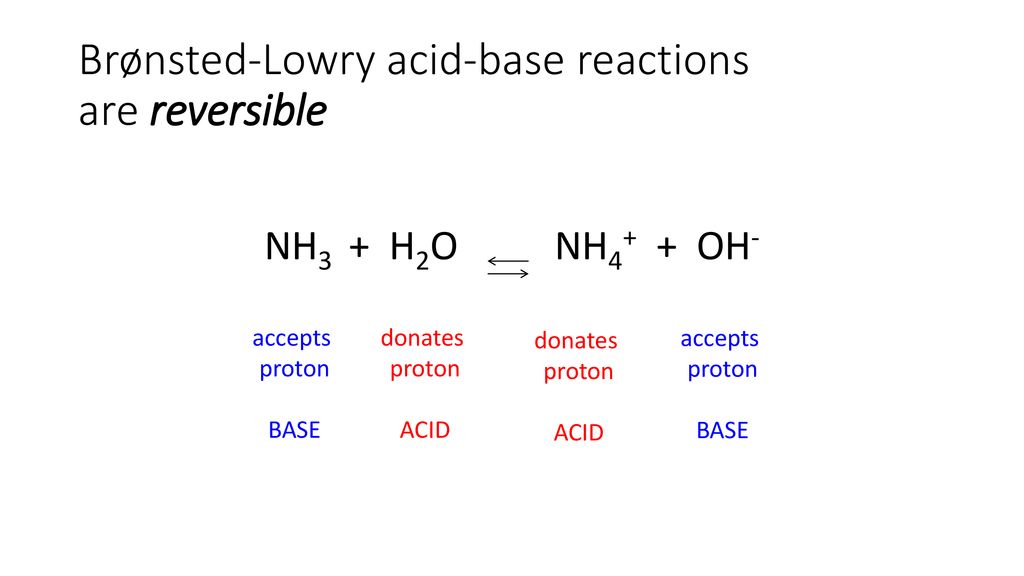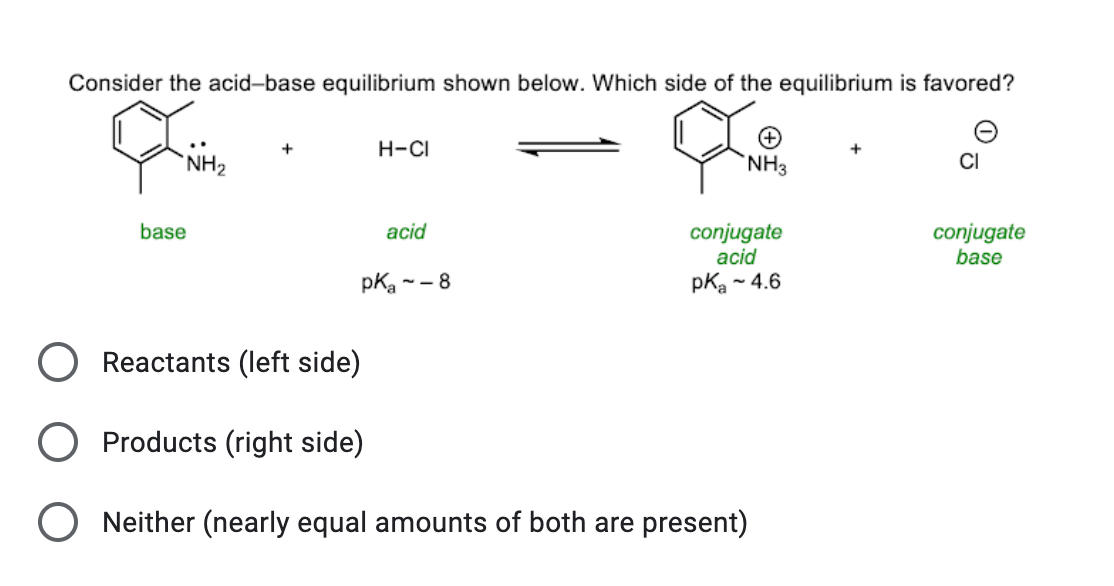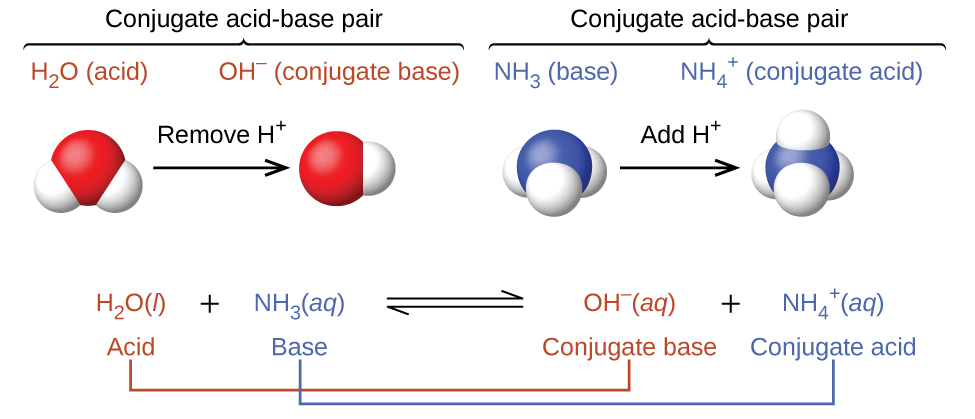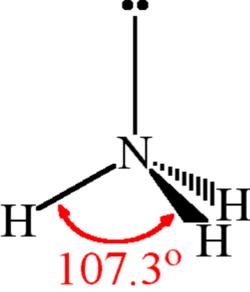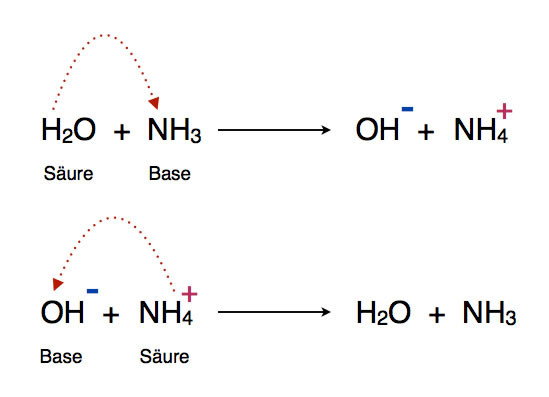
Welche Bedeutung hat die chemische Formel,,H2O+NH3- - - > OH- +NH4“ für die Säure-Base-Eigenschaften von Ammoniak? (Chemie, Säure-Base-Reaktion)

The dissolution of ammonia gas in water does not obey Henry's law. On dissolving, a major portion o fammonia, molecules unite with H2O to form NH4OH molecules. NH4OH again dissociates into NH4^+
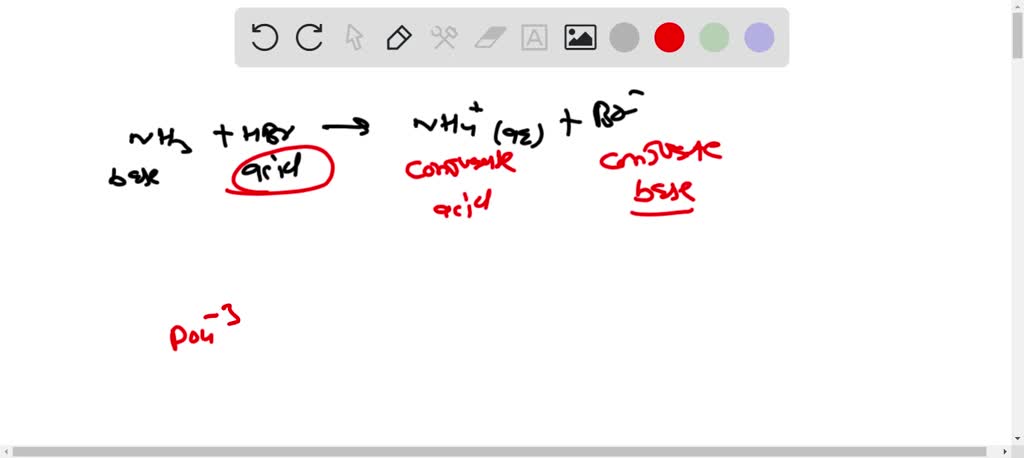
SOLVED: 16. Write an equation that shows the reaction of ammonia, NH3 with hydrobromic acid, HBr. Label the acid,the base, the conjugate acid, and the conjugate base. Write an equation that shows

Electric Field-Driven Acid−Base Chemistry: Proton Transfer from Acid (HCl) to Base (NH3/H2O) | The Journal of Physical Chemistry A

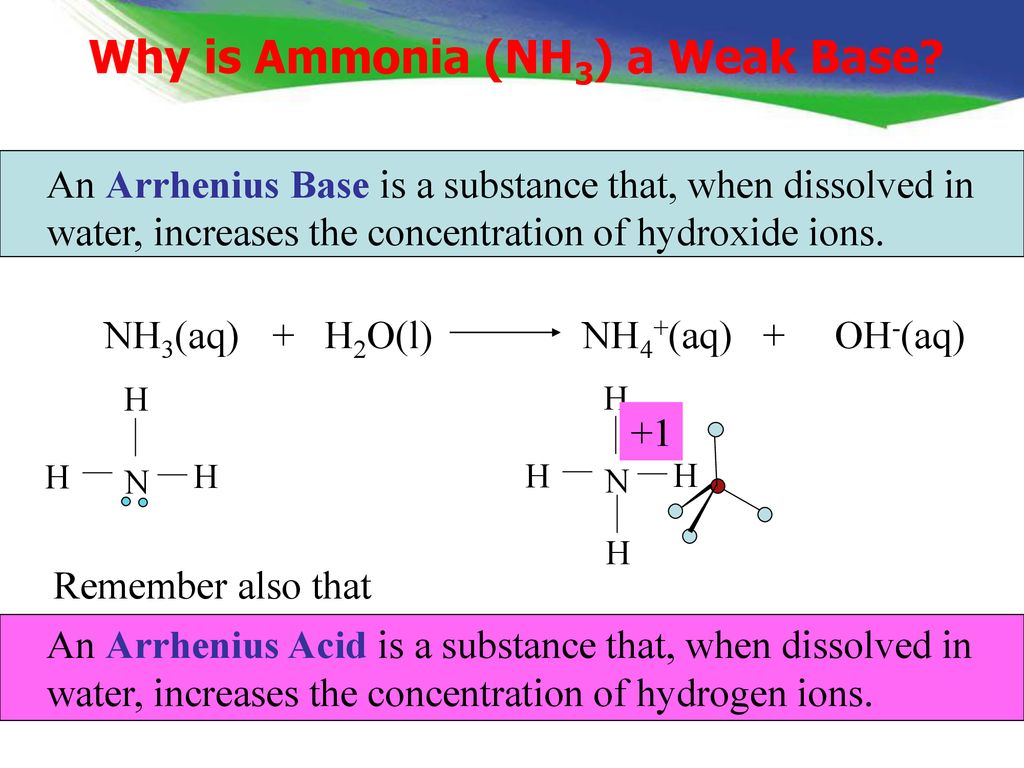


![Why does \\[N{H_3}\\] act as a Lewis base? Why does \\[N{H_3}\\] act as a Lewis base?](https://www.vedantu.com/question-sets/db470ea0-7477-412c-82d8-a4917a1132145281377342200076057.png)